In the rapidly advancing world of pharmaceuticals, safety has emerged as the cornerstone of progress. As new drugs and therapies revolutionize healthcare, ensuring their safety and effectiveness remains an ongoing challenge. This is where pharmacovigilance (PV)—the science of monitoring, assessing, and preventing adverse effects of medicines—plays a critical role.
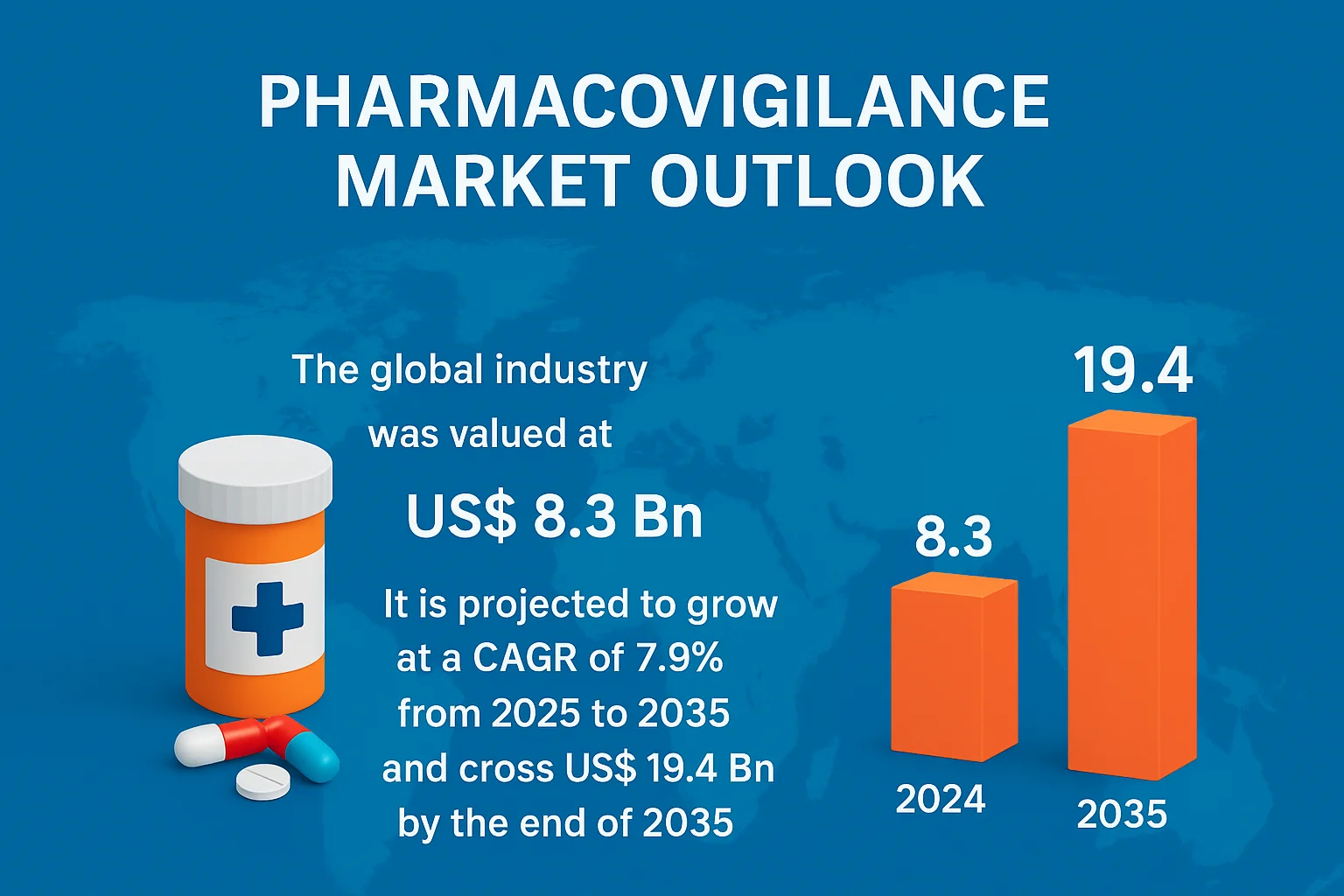
Valued at US$ 8.3 billion in 2024, the global pharmacovigilance market is projected to grow at a CAGR of 7.9% from 2025 to 2035, surpassing US$ 19.4 billion by 2035. This growth reflects the world’s growing commitment to patient safety, transparency, and regulatory compliance. Beyond being a regulatory necessity, pharmacovigilance has become a strategic pillar of drug development, helping companies build trust, reduce risks, and deliver safer medicines to millions.
Download Sample PDF Copy: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86696
What Is Pharmacovigilance and Why It Matters
Pharmacovigilance, derived from the Greek word pharmakon (drug) and Latin vigilare (to keep watch), refers to the continuous monitoring of drug safety after a product enters the market. It involves detecting, assessing, understanding, and preventing adverse drug reactions (ADRs) or any other medication-related problems.
While clinical trials are essential for testing the efficacy and safety of new drugs, they are often limited by controlled environments and small sample sizes. Once a drug is approved and used by diverse populations across varying conditions, unexpected side effects may emerge. Pharmacovigilance fills this gap by tracking real-world outcomes—identifying patterns, managing risks, and updating usage guidelines when necessary.
In essence, pharmacovigilance ensures that every pill, injection, or therapy prescribed to a patient is backed by continuous safety validation. It is the invisible force that keeps global healthcare systems accountable and patients protected.
The Global Push for Drug Safety
The increasing global dependence on pharmaceuticals, coupled with rapid drug innovation, has intensified the need for robust pharmacovigilance systems. The World Health Organization (WHO) emphasizes PV as a key component of public health, urging all countries to establish strong monitoring networks.
Every year, thousands of new drugs and biologics enter the global market. Many target complex diseases like cancer, autoimmune disorders, and rare genetic conditions. While these treatments offer hope, they also carry potential risks that only emerge after widespread use. As such, healthcare regulators worldwide—such as the U.S. FDA, European Medicines Agency (EMA), and Pharmacovigilance Risk Assessment Committee (PRAC)—have reinforced post-marketing safety surveillance as a mandatory process.
The goal is simple but vital: detect safety issues early, act decisively, and maintain patient trust.
Driving Forces Behind the Growth of Pharmacovigilance
The surge in pharmacovigilance demand can be attributed to several key global trends:
1. Rise in Global Drug Consumption and Chronic Diseases
The growing prevalence of chronic illnesses such as diabetes, cardiovascular disease, and cancer has led to higher medication use. As more patients rely on multiple drugs simultaneously, the likelihood of drug interactions and side effects increases—necessitating more robust pharmacovigilance systems.
2. Acceleration of Drug Development and Approvals
The pharmaceutical industry is moving faster than ever, with new treatments being developed and approved in record time. While this benefits patients, it also means less long-term safety data before commercialization. PV helps bridge this gap by continuously monitoring real-world outcomes once the drug is in circulation.
3. Expansion of Biologics and Personalized Medicine
Advanced therapies like monoclonal antibodies, gene therapies, and mRNA-based vaccines have complex mechanisms that require specialized monitoring. Pharmacovigilance teams now play a key role in tracking immune reactions, off-target effects, and long-term outcomes in these cutting-edge treatments.
4. Technological Integration in Safety Monitoring
Artificial Intelligence (AI), machine learning (ML), and big data analytics are transforming how pharmacovigilance operates. Automation enables faster data collection and adverse event reporting, while predictive algorithms can identify potential safety risks before they escalate. Cloud-based platforms now allow global data sharing and collaboration, improving response times across regulatory regions.
5. Strengthening of Global Regulations
Governments and health authorities have strengthened pharmacovigilance requirements to safeguard public health. The EU’s Good Pharmacovigilance Practices (GVP) and the U.S. FDA’s Postmarketing Safety Reporting Rule mandate continuous monitoring, transparent reporting, and real-time data submission for every approved drug. Non-compliance can lead to penalties, market withdrawals, and reputational damage.
Pharmacovigilance as a Strategic Advantage
Pharmaceutical and biotech companies are increasingly viewing pharmacovigilance not merely as a compliance function but as a strategic differentiator. Effective PV systems enhance corporate reputation, investor confidence, and market access.
Companies that invest in strong safety frameworks are more likely to gain regulatory trust and accelerate drug approvals. Moreover, a commitment to patient safety builds brand loyalty and public confidence—critical in an age where consumers demand transparency from healthcare providers.
Beyond compliance, pharmacovigilance also contributes to scientific innovation. By analyzing adverse event data, researchers gain valuable insights into drug mechanisms, interactions, and patient responses. These insights fuel the development of safer, more effective therapies and even identify new therapeutic uses for existing drugs.
The Role of Outsourcing and Collaboration
To manage growing data complexity and cost pressures, many pharmaceutical companies are outsourcing pharmacovigilance activities to Contract Research Organizations (CROs) and specialized service providers. Outsourcing enables access to advanced technology, skilled professionals, and global regulatory expertise.
Additionally, collaboration across borders is becoming essential. International pharmacovigilance partnerships—such as those between regulatory agencies, academic institutions, and the WHO—are helping create harmonized safety databases and standardized reporting frameworks. This global network ensures faster information exchange and collective action in response to safety alerts.
Looking Ahead: The Future of Drug Safety
The future of pharmacovigilance lies in automation, real-world data integration, and predictive analytics. AI-driven tools will make it possible to detect subtle patterns of adverse events long before they become widespread. Real-world evidence (RWE), derived from electronic health records and wearable devices, will provide a more complete picture of how drugs perform in everyday use.
Moreover, patient engagement will play a growing role. With mobile apps and digital health platforms, individuals can report side effects directly—empowering them to participate actively in drug safety.
By 2035, as the global pharmacovigilance market surpasses US$ 19 billion, the industry will evolve from reactive reporting to proactive prevention. Pharmacovigilance will stand at the heart of global healthcare innovation—bridging science, safety, and trust.
Conclusion
Pharmacovigilance is more than a regulatory requirement—it is the ethical backbone of modern medicine. It ensures that innovation in drug development never compromises patient well-being. As the world races toward personalized medicine, biologics, and digital therapeutics, the role of pharmacovigilance will only grow stronger.
By combining technology, data, and human expertise, pharmacovigilance is shaping a future where drug safety is not just monitored—but assured. In doing so, it safeguards not only lives but also the most vital currency in healthcare: patient trust.