Market Overview:
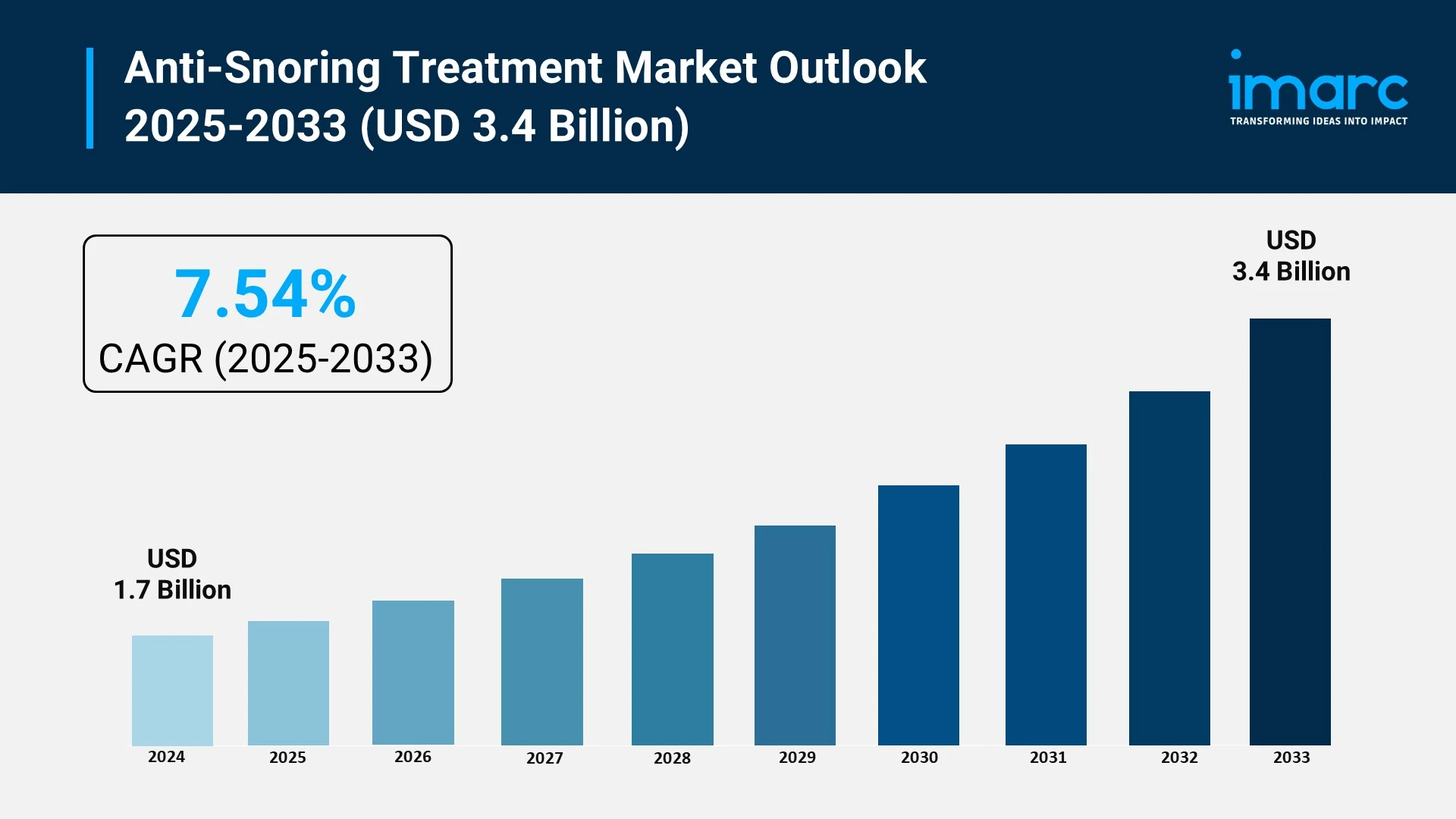
The anti-snoring treatment market is experiencing rapid growth, driven by rising prevalence of lifestyle-related sleep disorders, advancements in personalized oral appliance technology, and strategic government and healthcare initiatives. According to IMARC Group's latest research publication, "Anti-Snoring Treatment Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global anti-snoring treatment market size reached USD 1.7 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 3.4 Billion by 2033, exhibiting a growth rate (CAGR) of 7.54% during 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Download a sample PDF of this report: https://www.imarcgroup.com/anti-snoring-treatment-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends and Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Growth Factors in the Anti-Snoring Treatment Market
- Rising Prevalence of Lifestyle-Related Sleep Disorders
The primary catalyst for market expansion is the increasing global incidence of obstructive sleep apnea and chronic snoring, which are closely linked to rising obesity rates and an aging population. According to the World Health Organization, more than 1 billion adults worldwide are living with obesity, a condition that significantly increases the likelihood of airway obstruction during sleep due to excess tissue in the throat. In the United States alone, modeling indicates a 26.7% increase in adult sleep apnea prevalence within the current five-year period, creating a vast patient base requiring intervention. Furthermore, the geriatric population, particularly those between 55 and 60 years of age, remains the most affected demographic, as muscle tone in the upper airway naturally diminishes with age. This growing disease burden has shifted snoring from a social nuisance to a recognized medical priority, driving high demand for effective clinical treatments.
- Advancements in Personalized Oral Appliance Technology
A significant shift toward customized treatment solutions is fueling the adoption of oral appliances, which currently hold nearly 40% of the total market share. The integration of 3D digital scanning and 3D printing allows manufacturers like Glidewell and Panthera Dental to produce mandibular advancement devices that are tailored precisely to a patient's unique oral anatomy. These personalized appliances offer superior comfort and efficacy compared to traditional "boil-and-bite" alternatives, addressing the common issue of patient non-compliance. In 2024, Glidewell introduced the Silent Nite 3D Sleep Appliance specifically to target this need for precision. By providing a less invasive and more portable alternative to Continuous Positive Airway Pressure machines, these custom-fitted devices have become the preferred choice for patients with mild to moderate sleep apnea, effectively lowering the barriers to consistent nightly therapy and long-term health management.
- Strategic Government and Healthcare Initiatives
Growth is further supported by proactive government health policies and specialized medical initiatives aimed at improving diagnosis rates for sleep-related breathing disorders. Many healthcare systems are now prioritizing early intervention to mitigate the long-term costs associated with untreated snoring, such as hypertension, type 2 diabetes, and cardiovascular disease. For instance, the 31st Annual Advances in Diagnosis and Treatment of Sleep Apnea and Snoring, held in early 2026, highlights the ongoing professional commitment to integrating new screening protocols into primary care. In several regions, regulatory bodies have cleared over-the-counter versions of advanced devices to shorten the cycle from diagnosis to therapy. The FDA’s clearance of products like the Anti-snore Mouth Guard+ and the eXciteOSA daytime therapy device exemplifies this regulatory support, making clinical-grade solutions more accessible to the public without the immediate necessity of a specialist prescription or a hospital-based sleep study.
Key Trends in the Anti-Snoring Treatment Market
- Integration of AI and Real-Time Monitoring
The most prominent trend in the current market is the evolution of "smart" anti-snoring devices equipped with artificial intelligence and Bluetooth connectivity. Modern wearables, such as the Happy Ring cleared by the FDA in late 2024, utilize sophisticated sensors to detect acoustic snoring signals and physiological changes in real-time. These devices can provide immediate feedback through gentle vibrations that prompt users to change their sleeping positions without waking them. AI-driven mobile applications now analyze nightly data to offer personalized coaching, helping users understand how lifestyle factors like alcohol consumption or stress levels impact their snoring severity. Major industry leaders like ResMed have invested heavily in these digital ecosystems, leveraging generative AI assistants to guide patients through mask fittings and routine adjustments, turning traditional medical hardware into comprehensive, data-driven health management platforms.
- Surge in Direct-to-Consumer and Online Retail
The distribution landscape is undergoing a rapid transformation as e-commerce becomes the fastest-growing channel for anti-snoring solutions. Consumers are increasingly bypassing traditional clinical routes in favor of online pharmacies and direct-to-consumer storefronts, which offer a wider variety of products and the convenience of home delivery. This trend is particularly strong in the Asia-Pacific region, where urban populations are utilizing digital platforms to access global brands that may not be available in local physical retail outlets. Companies are capitalizing on this by offering "at-home" impression kits for custom devices, allowing patients to mail back their molds and receive laboratory-grade appliances via post. The online segment is projected to account for a significant portion of market transactions by 2030, driven by the availability of peer reviews, educational webinars, and lower price points compared to hospital-based procurement.
- Shift Toward Non-Invasive Daytime Therapies
A disruptive trend is the emergence of daytime neuromuscular stimulation as a preventative measure for nighttime snoring. Unlike traditional devices that must be worn during sleep, products like eXciteOSA involve a short daily session—typically 20 minutes—where mild electrical stimulation is used to tone the tongue and upper airway muscles. Real-world data from the United States shows high patient adherence to this therapy, with over 80% of users maintaining the routine during the initial weeks of treatment. This shift addresses the "compliance gap" often associated with bulky masks or uncomfortable mouthpieces. By strengthening the airway during waking hours, these therapies aim to prevent the collapse of throat tissues during the night. This innovation is gaining traction among "tech-savvy" and wellness-oriented consumers who prefer a discreet, device-free sleep experience over traditional mechanical interventions
Leading Companies Operating in the Global Anti-Snoring Treatment Industry:
- Airway Management Inc.
- Apnea Sciences Corporation
- Fisher & Paykel Healthcare Corporation Limited
- Koninklijke Philips N.V.
- MEDiTAS Ltd.
- Medtronic plc
- Mitsui Chemicals Inc.
- ResMed Inc.
- Rotech Healthcare Inc.
- SomnoMed
- The Pure Sleep Company
- Tomed GmbH
Anti-Snoring Treatment Market Report Segmentation:
By Device Type:
- Mandibular Advancement Devices (MAD)
- Tongue Stabilizing Devices (TSD)
- Continuous Positive Airway Pressure (CPAP) Devices
- Others
Continuous positive airway pressure (CPAP) devices represent the largest segment as they work by delivering a steady stream of air through a mask to keep the airway open during sleep.
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online
- Others
Hospital pharmacies account for the majority of the market share. They offer a wide range of anti-snoring treatment medications.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America's dominance in the anti-snoring treatment market is attributed to the rising number of individuals suffering from snoring complications.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302