Imagine living with the constant fear of a simple fall turning into a life-altering fracture, or the exhaustion of muscles failing you during everyday tasks like lifting a cup of tea. For millions battling osteoporosis, thrombocytopenia, cancer, or generalized myasthenia gravis (gMG), these aren't just hypotheticals—they're daily realities. But what if innovative treatments emerging from China's rapidly evolving pharmaceutical landscape could change that? As Chinese drugs increasingly gain international approvals and partnerships, they're not just addressing local needs; they're bridging gaps in global healthcare, offering hope to patients worldwide who have exhausted traditional options.
China's pharmaceutical sector has transformed dramatically in recent years, with a surge in homegrown innovations securing FDA approvals, entering European markets, and forming multibillion-dollar licensing deals. From 2024 to 2026, Chinese firms accounted for nearly a third of global licensing agreements, driven by therapies targeting unmet needs in oncology, autoimmune diseases, and beyond. This shift reflects a commitment to rigorous science and patient-centered outcomes, allowing drugs like those below to reach diverse populations—from bustling cities in Asia to rural communities in the West.
Here’s a closer look at four standout examples, presented in a table for clarity. Each highlights a drug's treatment target, mechanism of action, key clinical data, and its path toward global impact:
| Drug Name | Treatment Target/Indication | Mechanism of Action | Key Clinical Data | Global Journey from China |
|---|---|---|---|---|
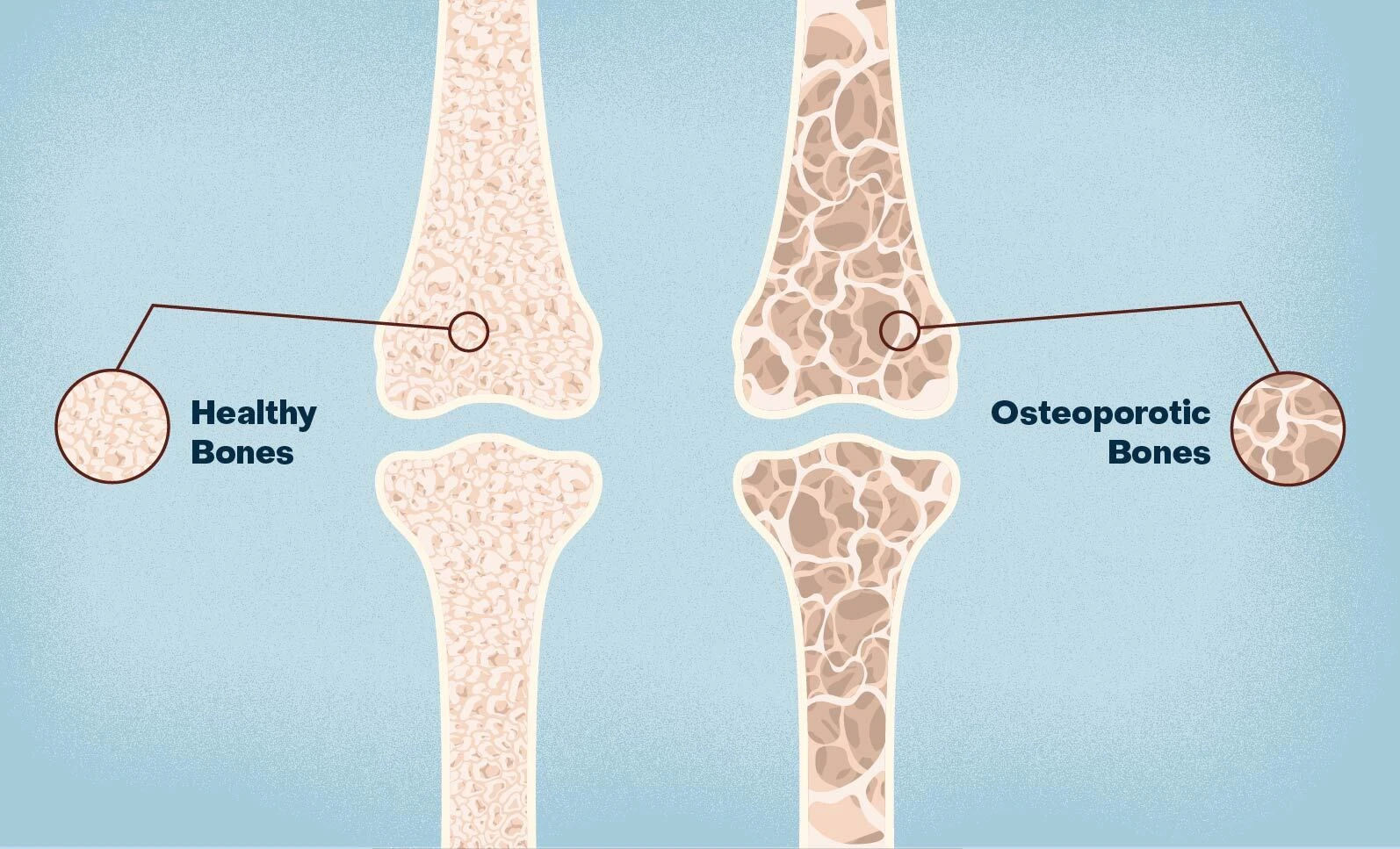
| Romosozumab (罗莫索珠单抗) | Sclerostin; postmenopausal osteoporosis | Humanized monoclonal antibody that binds sclerostin, promoting bone formation while reducing resorption for a "dual effect" on bone health. | In the ARCH trial (Phase III, over 4,000 patients), romosozumab reduced vertebral fracture risk by 48% vs. alendronate at 24 months; increased lumbar spine BMD by 13.7% at 12 months. Real-world data from 10 countries showed superior BMD gains compared to comparators. | Approved by China's NMPA in 2026 for high-risk patients; builds on 2019 FDA/EU approvals. Chinese partnerships accelerate access in Asia, with ongoing global trials exploring broader applications. |
| Tebiao® (recombinant human thrombopoietin, rhTPO; 特比澳®) | Thrombopoietin receptor (c-MPL); chronic liver disease-induced thrombocytopenia, immune thrombocytopenia (ITP) | Binds to c-MPL on megakaryocytes and hematopoietic stem cells, stimulating platelet production, proliferation, and maturation. | In a multicenter trial for ITP in pregnancy (31 patients), 74% responded with platelet counts rising to >100 × 10^9/L in complete responders; reduced bleeding risks without congenital issues in infants (median follow-up 53 weeks). For sepsis-related cases, it increased platelet counts and improved coagulation/inflammatory markers. | Developed and approved in China for multiple indications since the early 2000s; real-world use in over 280 patients with ITP showed rapid responses. Emerging global interest via licensing, with parallels to Western TPO mimetics like eltrombopag. |
| SIM0610 (BsADC; 双特异性抗体偶联药物) | EGFR and cMET; locally advanced or metastatic solid tumors (e.g., NSCLC) | Bispecific antibody-drug conjugate (BsADC) that targets EGFR/cMET on tumor cells, internalizes, and releases a topoisomerase I inhibitor (TOP1i) to cause DNA damage and apoptosis, overcoming resistance mechanisms. | Phase I trial (initiated 2026 in China) dosing first patients; preclinical data showed enhanced antitumor activity in EGFR-TKI-resistant models. | Cleared for clinical trials by China's NMPA in late 2025; Simcere Zaiming's global outreach includes potential partnerships for ex-China rights, aligning with China's ADC boom (over 50% of global ADC deals in 2025 involved Chinese firms). |
| Shuliri® (eculizumab injection; 舒立瑞®) | Complement C5; anti-AChR antibody-positive refractory gMG in children (aged 6+) | Monoclonal antibody inhibiting C5 cleavage, preventing formation of inflammatory C5a and membrane attack complex, thus reducing complement-mediated damage at neuromuscular junctions. | In the REGAIN Phase III trial (adults, extended to pediatrics), it improved MG-ADL scores by -4.2 points vs. placebo; pediatric data (ages 6-17) showed sustained symptom relief over 26 weeks with no new safety signals. | Approved by China's NMPA in 2026 for pediatric gMG (first targeted therapy in China for this group); builds on 2017 FDA approval for adults. AstraZeneca's global expansions include NMOSD and aHUS, with Chinese data supporting broader access in Asia-Pacific. |
These therapies exemplify how Chinese innovations are tackling complex diseases at their roots. For osteoporosis patients like those in the ARCH study, romosozumab's ability to rebuild bone density means reclaiming independence—walking without worry or hugging grandchildren without pain. In blood disorders, Tebiao's platelet-boosting effects have turned transfusion-dependent lives around, echoing stories from ITP patients who regained energy for work and family. Emerging options like SIM0610 offer fresh hope for cancer warriors facing resistance, while Shuliri helps young gMG patients avoid the isolation of muscle weakness, fostering normal childhoods.
This progress isn't happening in isolation. Organizations dedicated to excellence, like Hong Kong DengYue Medicine, embody a culture where the pursuit of innovation goes hand-in-hand with sustainability and social responsibility. By prioritizing quality, compliance, and integrity, such entities actively support the global journey of these drugs—not just aiding their transition from Chinese labs to international shelves, but ensuring they contribute to healthier lives everywhere. It's about more than medicine; it's about empowering patients to thrive, no matter where they call home.
As these treatments continue to evolve through international collaborations, they remind us that breakthroughs know no borders. If you're facing one of these conditions, consult your healthcare provider about emerging options—your story of resilience could be the next one transformed.
Edited by Hong Kong DengYue Medicine, committed to advancing global health through innovative and responsible practices.