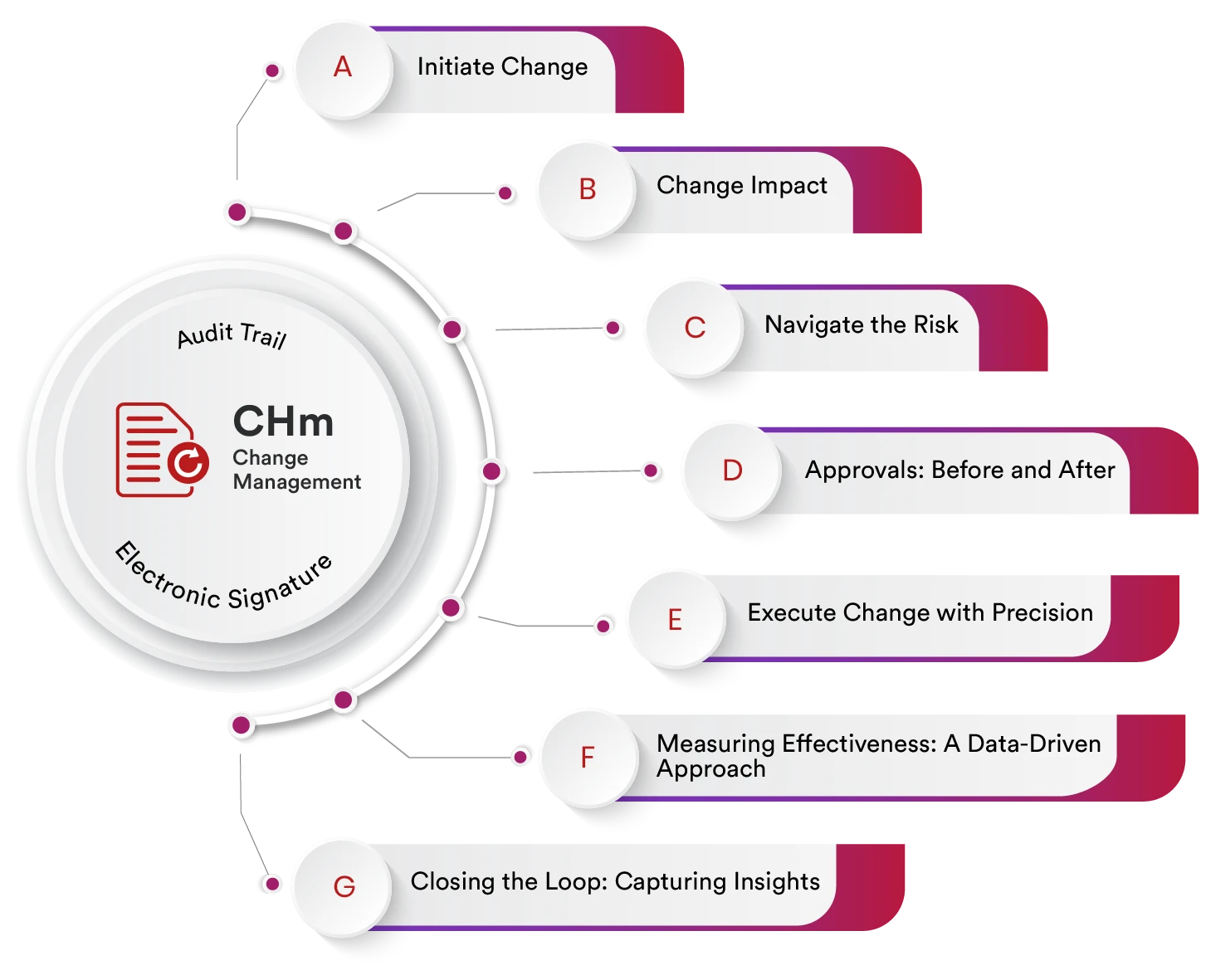
Change is inevitable in every organization. Whether updating SOPs, modifying product specifications, introducing new equipment, changing suppliers, or responding to audit findings, businesses must ensure every change is controlled, documented, and compliant.
Without a structured system, change processes become fragmented—leading to compliance gaps, product risks, and audit observations. This is where Qualityze Change Control Management Software helps organizations centralize, automate, and streamline change management across the enterprise.
What is Change Control Management Software?
Change Control Management Software is a digital solution that standardizes how changes are:
-
Requested
-
Reviewed
-
Risk-assessed
-
Approved
-
Implemented
-
Verified
It ensures complete traceability and compliance while reducing manual effort and operational risks.
For regulated industries, change control is not optional—it is a mandatory requirement under global standards and regulatory frameworks.
Why Change Control is Critical for Compliance
Regulatory bodies require documented and controlled change management processes. Improperly managed changes can lead to:
-
Regulatory warning letters
-
Product recalls
-
Customer complaints
-
Production delays
-
Financial losses
A structured change control system ensures that every change is evaluated for risk and impact before implementation.
Qualityze helps organizations align with standards such as:
-
FDA 21 CFR Part 11
-
ISO 9001
-
ISO 13485
-
IATF 16949
-
GMP and GxP guidelines
Key Capabilities of Qualityze Change Control Management Software
1. Centralized Change Request System
Submit and manage all change requests from a single cloud-based platform. Categorize changes as major, minor, or emergency and attach necessary documentation.
2. Configurable Approval Workflows
Automate multi-level approval processes with role-based routing. Reduce delays through automated notifications and reminders.
3. Risk and Impact Assessment
Evaluate potential risks before implementation. Assess impact on product quality, compliance, operations, and customer safety.
4. Integrated QMS Connectivity
Link change control with:
-
CAPA Management
-
Document Control
-
Audit Management
-
Deviation Management
-
Training Management
-
Risk Management
This ensures full traceability across quality processes.
5. Electronic Signatures & Audit Trail
Maintain secure, time-stamped audit trails with compliant electronic signatures for regulatory readiness.
6. Effectiveness Verification
Post-implementation reviews confirm whether the change achieved intended objectives without introducing new risks.
Benefits of Using Qualityze Change Control Software
Improved Regulatory Compliance
Ensure consistent documentation and inspection readiness at all times.
Reduced Operational Risk
Structured processes minimize errors, rework, and compliance failures.
Faster Change Cycle Time
Automation accelerates approvals and reduces manual tracking.
Real-Time Visibility
Dashboards and reporting tools provide full visibility into change status and trends.
Enterprise Scalability
Cloud-based architecture supports global teams and growing operations.
Industries That Benefit
Qualityze Change Control Management Software is ideal for:
-
Life Sciences
-
Pharmaceuticals
-
Medical Devices
-
Manufacturing
-
Automotive
-
Aerospace
-
Food & Beverage
These industries require strict governance and documented change control processes.
Why Choose Qualityze?
-
100% cloud-based platform
-
Highly configurable workflows
-
Built for regulated industries
-
Seamless integration across QMS modules
-
Secure and scalable architecture
-
Data-driven insights and analytics
Qualityze empowers organizations to move from reactive change management to proactive risk control.
Final Thoughts
Effective change control is the foundation of compliance and operational excellence. Organizations that rely on manual systems or disconnected tools face higher risks and inefficiencies.
With Qualityze Change Control Management Software, businesses can confidently manage changes, maintain regulatory compliance, and drive continuous improvement.
Modernize your change control process today and transform compliance into a competitive advantage.