Europe Photodynamic Therapy Market Overview
According To Renub Research Europe photodynamic therapy market is witnessing steady expansion, driven by growing demand for minimally invasive treatment options and continuous innovation in medical technologies. Photodynamic therapy, commonly known as PDT, is increasingly being adopted across oncology, dermatology, and cosmetic applications due to its precision, safety profile, and ability to selectively target diseased cells. Between 2025 and 2033, the market is forecast to grow significantly, supported by rising disease prevalence, aging populations, and a strong emphasis on non-surgical therapies within European healthcare systems.
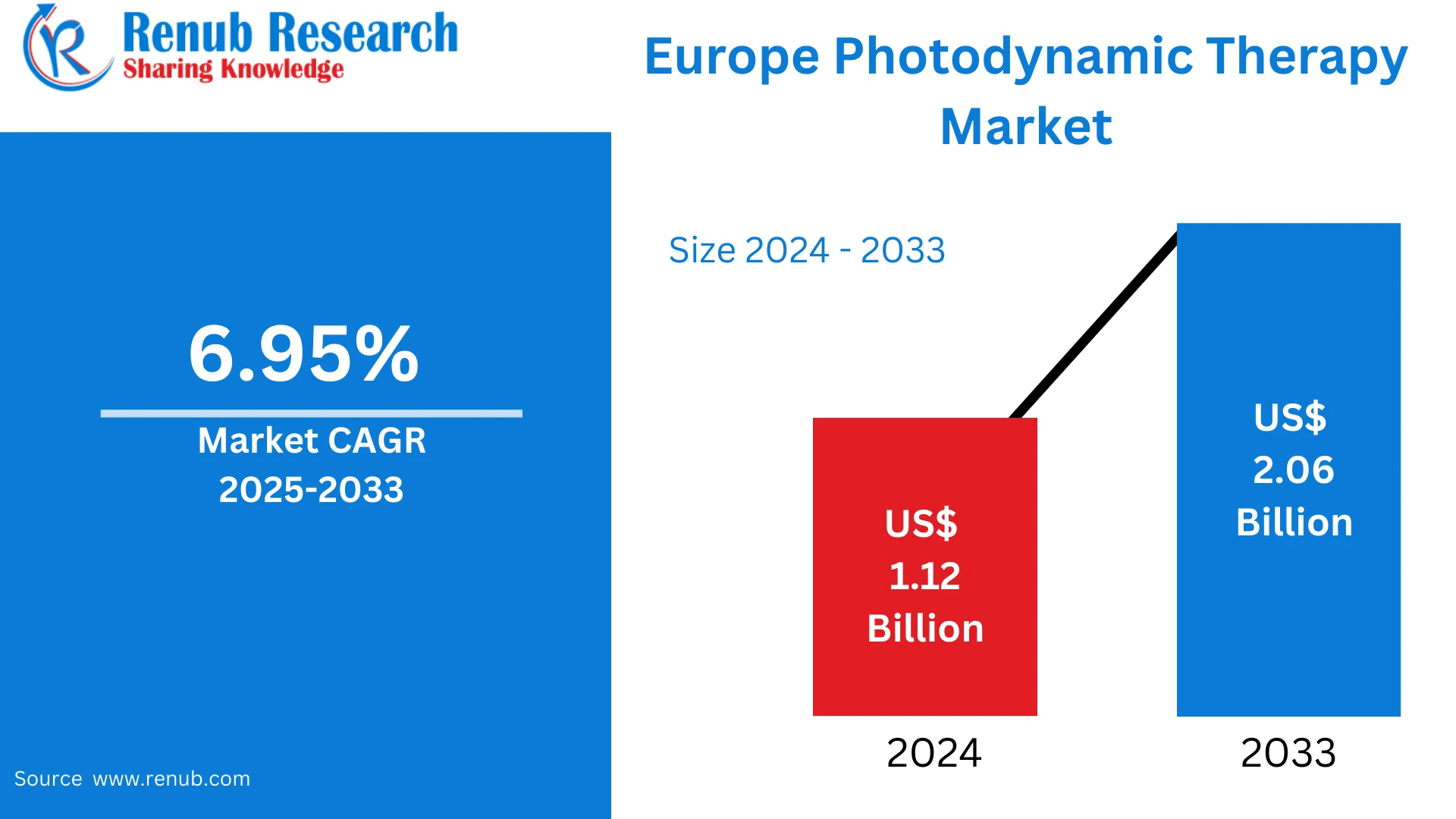
By 2025, the Europe photodynamic therapy market is expected to reach approximately US$ 1.12 billion, with projections indicating growth to around US$ 2.06 billion by 2033. This represents a compound annual growth rate of nearly 6.95% over the forecast period. The market’s upward trajectory reflects increasing acceptance of PDT among clinicians and patients, alongside advancements in photosensitizers and light-based devices that enhance treatment outcomes and expand clinical indications.
Europe Photodynamic Therapy Market Outlook
Photodynamic therapy is a minimally invasive medical procedure that combines a photosensitizing agent with a specific wavelength of light to eliminate abnormal or diseased cells. Once activated by light, the photosensitizer generates reactive oxygen species that destroy targeted cells while preserving surrounding healthy tissue. This mechanism makes PDT particularly suitable for treating surface-level and localized conditions.
In Europe, PDT is widely used for skin-related disorders such as actinic keratosis, basal cell carcinoma, and certain precancerous lesions. It is also gaining traction in the treatment of internal malignancies, including lung and esophageal cancers. Beyond oncology, photodynamic therapy has established applications in dermatology for acne management and cosmetic skin rejuvenation, further broadening its market scope.
The outlook for the European PDT market remains positive, as healthcare providers increasingly prioritize treatments that offer clinical effectiveness with reduced recovery times, minimal scarring, and fewer systemic side effects. Growing awareness among patients and improvements in clinical evidence are strengthening confidence in PDT as a viable alternative or complement to traditional therapies.
Download Free Sample Report:https://www.renub.com/request-sample-page.php?gturl=europe-photodynamic-therapy-market-p.php
Growth Drivers in the Europe Photodynamic Therapy Market
Increasing Prevalence of Cancer and Skin Disorders
One of the primary drivers of the Europe photodynamic therapy market is the rising incidence of cancer and chronic skin conditions. Skin cancers such as basal cell carcinoma and actinic keratosis are becoming more common due to aging populations and prolonged exposure to ultraviolet radiation. Additionally, cancers of the lung, esophagus, and head and neck are contributing to an expanding patient pool that requires effective and targeted treatment solutions.
Photodynamic therapy offers an attractive option for these conditions by minimizing damage to healthy tissue and reducing treatment-related complications. Elderly patients, who are more susceptible to cancer and skin disorders, particularly benefit from PDT’s minimally invasive nature. As Europe continues to experience demographic aging, demand for safer and well-tolerated therapies is expected to increase, reinforcing market growth.
Technological Advancements in PDT Devices
Continuous innovation in light delivery systems, laser technologies, and photosensitizer formulations is significantly enhancing the effectiveness of photodynamic therapy. Modern PDT devices provide improved light penetration, greater precision, and shorter treatment durations, resulting in better patient outcomes and higher satisfaction levels.
Manufacturers across Europe are investing heavily in research and development to create next-generation devices that are more efficient, portable, and user-friendly. These advancements are making PDT increasingly accessible in hospitals, specialty clinics, and outpatient settings. As technology improves and costs gradually decline, adoption rates are expected to rise across both public and private healthcare facilities.
Growing Preference for Non-Invasive Treatments
Patients and healthcare professionals alike are showing a strong preference for non-invasive and minimally invasive treatment options. Photodynamic therapy aligns well with this trend by offering effective treatment with limited discomfort, rapid recovery, and reduced risk of complications.
Compared to surgery, chemotherapy, or radiation therapy, PDT provides a targeted approach that limits collateral damage and preserves cosmetic outcomes. This is particularly important in dermatology and early-stage cancer treatment, where quality of life considerations play a crucial role. The shift toward patient-centric care models in Europe is therefore accelerating the adoption of photodynamic therapy.
Challenges in the Europe Photodynamic Therapy Market
Limited Awareness and Clinical Acceptance
Despite its clinical benefits, photodynamic therapy still faces challenges related to limited awareness and acceptance among healthcare professionals. Many clinicians continue to rely on conventional treatment methods due to familiarity, established protocols, and broader training exposure. In some regions, insufficient education and training opportunities restrict the integration of PDT into routine clinical practice.
The lack of standardized treatment guidelines across European healthcare systems further hampers widespread adoption. Addressing these challenges will require greater emphasis on professional training, clinical evidence dissemination, and inclusion of PDT in treatment recommendations.
High Treatment Costs and Reimbursement Barriers
Cost remains a significant barrier to the widespread adoption of photodynamic therapy in Europe. The need for specialized equipment, proprietary photosensitizers, and trained personnel contributes to relatively high treatment expenses. In several European countries, reimbursement policies for PDT are inconsistent or poorly defined, limiting affordability for patients and healthcare providers.
Public healthcare systems with constrained budgets may be reluctant to invest in PDT infrastructure without clear reimbursement frameworks. Market growth could be further accelerated if pricing structures become more competitive and reimbursement pathways more transparent.
Europe Photodynamic Therapy Devices Market
The photodynamic therapy devices segment represents a substantial portion of the overall European market. This segment includes lasers, light-emitting diode systems, and fiber-optic light delivery devices used in conjunction with photosensitizing drugs. Hospitals and specialty clinics are increasingly investing in advanced PDT equipment to expand their treatment capabilities and improve clinical precision.
Technological improvements have led to the development of compact and portable devices, enabling use in outpatient clinics and, in some cases, homecare settings. As device efficiency improves and integration with digital health technologies increases, this segment is expected to remain a key revenue contributor.
Europe Cancer Photodynamic Therapy Market
Cancer treatment is the largest application segment within the Europe photodynamic therapy market. PDT is being increasingly adopted for the management of head and neck cancers, non-small cell lung cancer, and esophageal cancer. Its localized action and reduced systemic toxicity make it an appealing option, particularly for patients who may not tolerate aggressive therapies.
European oncology centers are integrating PDT into multimodal treatment strategies, using it alongside surgery, chemotherapy, or radiotherapy to enhance overall outcomes. Ongoing clinical research and expanding evidence bases are reinforcing the role of PDT in cancer care pathways.
Europe Psoriasis Photodynamic Therapy Market
Photodynamic therapy is gaining recognition as an effective option for managing moderate to severe psoriasis, especially in cases where traditional treatments have limited efficacy. European dermatologists are increasingly adopting PDT due to its targeted action, low systemic impact, and potential for long-term disease control.
The rising prevalence of autoimmune and lifestyle-related skin disorders across Europe is contributing to growing demand for advanced dermatological therapies. Government initiatives and funding aimed at improving chronic disease management further support the adoption of PDT in this segment.
Europe Photodynamic Therapy Hospitals Market
Hospitals represent a major end-user segment in the Europe photodynamic therapy market. Equipped with advanced infrastructure, skilled professionals, and access to reimbursement mechanisms, hospitals are well-positioned to deliver PDT services. The expansion of oncology and dermatology departments within both public and private hospitals is driving increased investment in photodynamic therapy systems.
Hospitals are also leveraging PDT to differentiate their service offerings, improve patient outcomes, and strengthen their technological capabilities. This trend is expected to sustain strong demand from the hospital segment throughout the forecast period.
Europe Photodynamic Therapy Cancer Treatment Centers Market
Specialized cancer treatment centers play a critical role in advancing photodynamic therapy adoption across Europe. These centers often lead clinical trials, adopt innovative photosensitizers early, and integrate PDT into comprehensive cancer care programs.
Their focus on research, patient education, and specialist training contributes significantly to the credibility and acceptance of PDT in oncology. As demand for non-surgical cancer treatments continues to grow, cancer treatment centers are expected to remain key drivers of market expansion.
Country-Level Insights in the Europe Photodynamic Therapy Market
Germany leads the European photodynamic therapy market, supported by a robust healthcare infrastructure, strong clinical adoption, and an active research environment. The country’s early integration of PDT into oncology and dermatology practices continues to sustain its leadership position.
The United Kingdom represents a growing market, driven by public healthcare coverage for certain PDT indications, strong clinical research activity, and increasing skin cancer incidence. Collaborative efforts between academic institutions and healthcare providers are further expanding clinical applications.
The Netherlands is emerging as an innovative market, particularly within academic and dermatological settings. Its emphasis on precision medicine and minimally invasive procedures aligns well with the advantages of photodynamic therapy.
Russia presents a mixed outlook, with adoption concentrated in urban hospitals and private centers. While awareness and reimbursement challenges persist, increasing healthcare investment and demand for advanced cancer treatments offer future growth potential.
Market Segmentation Overview
The Europe photodynamic therapy market is segmented by product type into photodynamic therapy devices and photosensitizer drugs. By application, it covers cancer, actinic keratosis, psoriasis, acne, and other indications. End users include hospitals, cosmetic and dermatology clinics, cancer treatment centers, and other healthcare facilities.
Geographically, the market spans France, Germany, Italy, Spain, the United Kingdom, Belgium, the Netherlands, Russia, Poland, Greece, Norway, Romania, Portugal, and the rest of Europe.
Competitive Landscape and Key Players
The competitive landscape of the Europe photodynamic therapy market is characterized by the presence of established pharmaceutical and medical device companies, along with emerging innovators. Market participants focus on product development, strategic collaborations, and geographic expansion to strengthen their market positions.
Companies operating in this space are typically evaluated across multiple dimensions, including business overview, leadership, recent developments, SWOT analysis, and revenue performance. Key players continue to invest in research, clinical trials, and partnerships to enhance their portfolios and meet evolving clinical needs.